Vitamin E is also known as Tocopherol. Alpha-tocopherol is traditionally recognized as the most active form of vitamin E in humans, and is a powerful antioxidant. The measurement of "vitamin E" activity in international units (IU) was based on fertility enhancement by the prevention of spontaneous abortions in pregnant rats relative to alpha tocopherol. It increases naturally to about 150% of normal in the maternal circulation during human pregnancies. The other isomers are slowly being recognized as research begins to elucidate their additional roles in the human body. Many naturopathic and orthomolecular medicine advocates suggest that vitamin E supplements contain at least 20% by weight of the other natural vitamin E isomers. Commercially available blends of natural vitamin E include "mixed tocopherols" and "high gamma tocopherol" formulas. Also selenium, Coenzyme Q10, and ample vitamin C have been shown to be essential cofactors of natural tocopherols.
Antioxidants such as vitamin E act to protect cells against the effects of free radicals, which are potentially damaging by-products of the body's metabolism. Free radicals can cause cell damage that may contribute to the development of cardiovascular disease and cancer. Vitamin C and other anti-oxidants recycle vitamin E end-products back into effective suppressors of free radicals. Studies are underway to determine whether vitamin E might help prevent or delay the development of those chronic diseases.
Vegetable oils, nuts, wheat germ and green leafy vegetables are the main dietary sources of vitamin E. Fortified breakfast cereals are also an important source of vitamin E in the United States. Although originally extracted from wheat germ oil, most natural vitamin E supplements are now derived from vegetable oils, usually soybean oil.
Commercial vitamin E supplements can be classified into several distinct categories: fully synthetic vitamin E, "d,l-alpha-tocopherol", the most inexpensive, most commonly sold supplement form usually as the acetate ester; semisynthetic "natural source" vitamin E esters, the "natural source" forms used in tablets and multiple vitamins; highly fractionated natural d-alpha tocopherol; less fractionated "natural mixed tocopherols"; high gamma-tocopherol fraction supplements; and tocotrienol supplements.
Synthetic vitamin E, usually marked as d,l-tocopherol or d,l tocopheryl acetate, with 50% d-alpha tocopherol moiety and 50% l-alpha-tocopherol moiety, as synthesized by an earlier process is now actually manufactured as all-racemic alpha tocopherol, with only about one alpha tocopherol molecule in 8 molecules as actual d-alpha tocpherol. The synthetic form is not as active as the natural alpha tocopherol form. The 1950's thalidomide disaster with numerous severe birth defects is a common example of d- vs l- epimer forms type problem with synthesized racemic mixtures. Information on any side effects of the synthetic vitamin E epimers is not readily available. Naturopathic and orthomolecular medicine advocates have long considered the synthetic vitamin E forms to be with little or no merit for cancer, circulatory and heart diseases.
Semisynthetic "natural source" vitamin E, manufacturers convert the common natural beta, gamma and delta tocopherol isomers into esters using acetic or succinic acid and add methyl groups to yield d-alpha tocopheryl esters such as d-alpha tocopheryl acetate or d-alpha tocopheryl succinate. These tocopheryl esters are more stable and are easy to use in tablets and multiple vitamin pills. Because only alpha tocopherols were officially counted as "vitamin E" in supplements, refiners and manufacturers faced enormous economic pressure to esterify and methylate the other natural tocopherol isomers, d-beta-, d-gamma- and d-delta-tocopherol into d-alpha tocopheryl acetate or succinate. In the healthy human body, the semisynthetic forms are easily de-esterified over several days, primarily in the liver, but not for common problems in aged or ill patients.
My chemistry class notes from high school, mainly relating to organic chemistry.
Saturday, September 03, 2005
Friday, July 29, 2005
Vitamin A
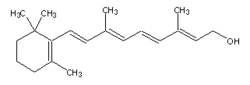
Vitamin A is also known as Retinol.
Retinol, vitamin A, is a fat-soluble, antioxidant vitamin important in vision and bone growth. It belongs to the family of chemical compounds known as retinoids. Retinol is ingested in a precursor form; animal sources (milk and eggs) contain retinyl esters, whereas plants (carrots, spinach) contain carotenoids. Tissue cells convert these precursors to retinol, and then to either retinal or retinoic acid.
Sunday, June 19, 2005
What is Salt?
In chemistry, a salt is a chemical compound composed of cations (positively charged ions) bound to anions (negatively charged ions). They are typically the product of a chemical reaction between a base and an acid, the base contributing the cation and the acid contributing the anion.
One example is table salt, in common usage often simply called salt. It is the specific salt sodium chloride, and is described thoroughly in that article. Its formula is NaCl and it is the product of the base sodium hydroxide, NaOH and hydrochloric acid, HCl. Table salt is the same as sea salt.
In general, salts are ionic compounds which form crystals. They are usually soluble in water, where the two ions separate. Salts typically have a high melting point, low hardness, and low compressibility. If molten or dissolved in water, they conduct electricity.
One example is table salt, in common usage often simply called salt. It is the specific salt sodium chloride, and is described thoroughly in that article. Its formula is NaCl and it is the product of the base sodium hydroxide, NaOH and hydrochloric acid, HCl. Table salt is the same as sea salt.
In general, salts are ionic compounds which form crystals. They are usually soluble in water, where the two ions separate. Salts typically have a high melting point, low hardness, and low compressibility. If molten or dissolved in water, they conduct electricity.
Thursday, May 19, 2005
Amino Acids
Amino acids are biochemical building blocks. They form short polymer chains called peptides or polypeptides which in turn form structures called proteins.
All twenty amino acids are encoded by the standard genetic code and are called proteinogenic or standard amino acids. Rarer, more complicated ones are produced by the body and are called nonstandard. Proline is the only proteinogenic amino acid whose side group is cyclic and links to the a-amino group, forming a secondary amino group. Formerly, proline was misleadingly called an imino acid. Other amino acids contained in proteins are usually formed by post-translational modification, that is modification after translation (protein synthesis). These modifications are often essential for the function of the protein. At least two amino acids other than the standard 20 are sometimes incorporated into proteins during translation:
Selenocysteine is incorporated into some proteins at a UGA codon, which is normally a stop codon.
Pyrrolysine is used by some methanogens in enzymes that they use to produce methane. It is coded for similarity to selenocysteine but with the codon UAG instead.
Although only 20 amino acids are genetically coded, over 100 have been found in nature. Some of these have been detected in meteorites, especially in a type known as carbonaceous chondrites. Microorganisms and plants often produce very uncommon amino acids, which can be found in peptidic antibiotics (e.g. nisin or alamethicin). Lanthionine is a sulfide-bridged alanine dimer which is found together with unsaturated amino acids in lantibiotics (antibiotic peptides of microbial origin). 1-Aminocyclopropane-1-carboxylic acid (ACC) is a small disubstituted cyclic amino acid and a key intermediate in the production of the plant hormone ethylene.
In addition to amino acids for protein synthesis, there are other biologically important amino acids, such as the neurotransmitters glycine, GABA and glutamate, as well as carnitine (used in lipid transport within a cell), ornithine, citrulline, homocysteine, hydroxyproline, hydroxylysine, and sarcosine.
Some of the 20 standard amino acids are called essential amino acids, because they cannot be synthesized by the body from other compounds through chemical reactions, but instead must be taken in with food. In humans, the essential amino acids are lysine, leucine, isoleucine, methionine, phenylalanine, threonine, tryptophan, valine, and (in children) histidine and arginine.
All twenty amino acids are encoded by the standard genetic code and are called proteinogenic or standard amino acids. Rarer, more complicated ones are produced by the body and are called nonstandard. Proline is the only proteinogenic amino acid whose side group is cyclic and links to the a-amino group, forming a secondary amino group. Formerly, proline was misleadingly called an imino acid. Other amino acids contained in proteins are usually formed by post-translational modification, that is modification after translation (protein synthesis). These modifications are often essential for the function of the protein. At least two amino acids other than the standard 20 are sometimes incorporated into proteins during translation:
Selenocysteine is incorporated into some proteins at a UGA codon, which is normally a stop codon.
Pyrrolysine is used by some methanogens in enzymes that they use to produce methane. It is coded for similarity to selenocysteine but with the codon UAG instead.
Although only 20 amino acids are genetically coded, over 100 have been found in nature. Some of these have been detected in meteorites, especially in a type known as carbonaceous chondrites. Microorganisms and plants often produce very uncommon amino acids, which can be found in peptidic antibiotics (e.g. nisin or alamethicin). Lanthionine is a sulfide-bridged alanine dimer which is found together with unsaturated amino acids in lantibiotics (antibiotic peptides of microbial origin). 1-Aminocyclopropane-1-carboxylic acid (ACC) is a small disubstituted cyclic amino acid and a key intermediate in the production of the plant hormone ethylene.
In addition to amino acids for protein synthesis, there are other biologically important amino acids, such as the neurotransmitters glycine, GABA and glutamate, as well as carnitine (used in lipid transport within a cell), ornithine, citrulline, homocysteine, hydroxyproline, hydroxylysine, and sarcosine.
Some of the 20 standard amino acids are called essential amino acids, because they cannot be synthesized by the body from other compounds through chemical reactions, but instead must be taken in with food. In humans, the essential amino acids are lysine, leucine, isoleucine, methionine, phenylalanine, threonine, tryptophan, valine, and (in children) histidine and arginine.
Saturday, April 16, 2005
Chemistry
Chemistry (derived from alchemy) is the science of matter at or near the atomic scale. Such matter includes atoms and the collections of atoms (such as molecules, crystals, and metals) that constitute materials encountered in everyday life. Chemistry deals with the composition and properties of such structures, as well as their transformations and interactions. According to modern chemistry, the physical properties of materials are generally predictable from their structure at the atomic scale. Chemistry is, along with physics, one of the most fundamental natural sciences.
Subscribe to:
Comments (Atom)