Kitchen Table Chemistry
My chemistry class notes from high school, mainly relating to organic chemistry.
Friday, April 05, 2013
Iron in my diet
Rich sources of dietary iron are red meat, lentils, beans, poultry, fish, leaf vegetables, watercress, tofu, chickpeas, black-eyed peas, blackstrap molasses, fortified bread, and fortified breakfast cereals. Iron in low amounts is found in molasses, teff and farina. Iron in meat (heme iron) is more easily absorbed than iron in vegetables. Although some studies suggest that heme/hemoglobin from red meat has effects which may increase the likelihood of colorectal cancer, there is still some controversy, and even a few studies suggesting that there is not enough evidence to support such claims.
Iron provided by dietary supplements is often found as iron(II) fumarate, although iron sulfate is cheaper and is absorbed equally well. Elemental iron, or reduced iron, despite being absorbed at only one third to two thirds the efficiency (relative to iron sulfate), is often added to foods such as breakfast cereals or enriched wheat flour. Iron is most available to the body when chelated to amino acids and is also available for use as a common iron supplement. Often the amino acid chosen for this purpose is the cheapest and most common amino acid, glycine, leading to "iron glycinate" supplements. The Recommended Dietary Allowance (RDA) for iron varies considerably based on age, gender, and source of dietary iron (heme-based iron has higher bioavailability). Infants may require iron supplements if they are bottle-fed cow's milk. Blood donors and pregnant women are often advised to supplement their iron intake.
Thursday, September 15, 2011
Mole (Chemical Unit)
The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities(e.g., atoms, molecules, ions, electrons) as there are atoms in 12 grams of pure carbon-12 (12C), the isotope of carbon with atomic weight 12. This corresponds to a value of6.02214179(30)×1023 elementary entities of that substance. It is one of the base units in the International System of Units, and has the unit symbol mol.
The mole is widely used in chemistry, instead of units of mass or volume, as a convenient way to express the amounts of reagents and products of chemical reactions. For example, the chemical equation 2 H2 + O2 → 2 H2O implies that 2 mol of dihydrogen and 1 mol of dioxygen react to form 2 mol of water. The mole may also be used to express the number of atoms, ions, or other elementary entities in some sample. The concentration of a solution is commonly expressed by its molarity, the number of moles of the dissolved substance per litre of solution.
The number of molecules in a mole (known as Avogadro's number) is defined so that the mass of one mole of a substance, expressed in grams, is exactly equal to the substance's mean molecular weight. For example, the mean molecular weight of natural water is about 18.015, so one mole of water is about 18.015 grams. This property considerably simplifies many chemical and physical computations.
The name gram-molecule was formerly used for essentially the same concept. The name gram-atom (abbreviated gat.) has been used for related but distinct concept, namely a quantity of a substance that contains Avogadro's number of atoms, whether isolated or combined in molecules. Thus, for example, 1 mole of MgB2 is 1 gram-molecule of MgB2 but 3 gram-atoms of MgB2.
Saturday, February 27, 2010
Cod Liver Oil
Cod liver oil and fish oil are similar, but cod liver oil has much higher levels of vitamins A and D. Depending on the quality of the oil, the flavor and aroma range from a mild sardine-like flavor, to an intense odor of fish. High quality cod liver oil is a pale-yellow, thin, oily liquid, having a slightly fishy and bland taste. Manufacturers sometimes add flavorings, such as citrus or mint essence, to cod liver oil to make it more palatable. Cod liver oil is widely taken to ease the pain and joint stiffness associated with arthritis, and has also been clinically proven to have a positive effect on heart, bone, and brain, as well as helping to nourish skin, hair, and nails. Because cod liver oil has a very high level of Vitamin A, it is possible to exceed the Recommended Dietary Allowance (RDA) of vitamin A. Vitamin A accumulates in body fat, and can reach harmful levels sufficient to cause hypervitaminosis A. Pregnant women may want to consider consulting a doctor when taking cod liver oil because of the high amount of natural forms of Vitamin A (Retinol). High doses of synthetic Vitamin A (Retinoids) have been shown in some cases to cause birth defects.
Friday, October 03, 2008
Alpha Lipoic Acid
Alpha lipoic acid is one of the most potent antioxidants known. It is found in the body in the liver and in the blood and is known to help maintain nerve function. Its main characteristic as an antioxidant is that it functions as both a water soluble and fat soluble antioxidant that is effective against a broader range of free radicals.
Lipoic acid is found in a variety of foods, notably kidney, heart and liver meats as well as spinach, broccoli and potatoes. Alpha-lipoic acid (ALA) is an effective antioxidant and prevents the symptoms of vitamin C and vitamin E deficiency. It is able to scavenge reactive species in vitro, though there is little or no evidence that this actually occurs in vivo. The relatively good scavenging activity of lipoic acid is due to the strained conformation of the 5-membered ring in the intramolecular disulfide.
Lipoic acid is found in a variety of foods, notably kidney, heart and liver meats as well as spinach, broccoli and potatoes. Alpha-lipoic acid (ALA) is an effective antioxidant and prevents the symptoms of vitamin C and vitamin E deficiency. It is able to scavenge reactive species in vitro, though there is little or no evidence that this actually occurs in vivo. The relatively good scavenging activity of lipoic acid is due to the strained conformation of the 5-membered ring in the intramolecular disulfide.
Friday, August 01, 2008
Low Carbohydrate Diet
Low carb diets, like the Atkin's diet have been around for a long time. As well as the Atkin's diet, low carbohydrate levels is the basis for a number of diet plans. The different types do have minor variations but all are basically low carb diets.
If foods high in digestible carbohydrates (e.g. breads, pasta) are consumed they are usually limited or replaced with foods containing a higher percentage of proteins and fats (e.g. meats, soy products) and often other foods low in carbohydrates (e.g. green leafy vegetables).
In the 1990s Dr. Atkins published Dr. Atkins New Diet Revolution and other doctors began to publish books based on the same principles. This has been said to be the beginning of the "low carb craze." During the late 1990s and early 2000s low-carbohydrate diets became some of the most popular diets in the U.S. (by some accounts as much as 18% of the population was using a low-carbohydrate diet at its peak) and spread to many countries. These were, in fact, noted by some food manufacturers and restaurant chains as substantially affecting their businesses (notably Krispy Kreme, and many fast food chains). This was in spite of the fact that the mainstream medical community continued to denounce low-carbohydrate diets as being a dangerous trend. It is, however, valuable to note that many of these same doctors and institutions at the same time quietly began altering their own advice to be closer to the low-carbohydrate recommendations (e.g. eating more protein, eating more fiber/less starch, reducing consumption of juices by children). The low carbohydrate advocates did some adjustments of their own increasingly advocating controlling fat and eliminating trans fat. Many of the diet guides and gurus that appeared at this time intentionally distanced themselves from Atkins and the term low carb (because of the controversies) even though their recommendations were based on largely the same principles (e.g. the Zone diet). As such it is often a matter of debate which diets are really low-carbohydrate and which are not. The 1990s and 2000 also saw the publication of an increased number of clinical studies regarding the effectiveness and safety (pro's and con's) of low-carbohydrate diets.
If foods high in digestible carbohydrates (e.g. breads, pasta) are consumed they are usually limited or replaced with foods containing a higher percentage of proteins and fats (e.g. meats, soy products) and often other foods low in carbohydrates (e.g. green leafy vegetables).
In the 1990s Dr. Atkins published Dr. Atkins New Diet Revolution and other doctors began to publish books based on the same principles. This has been said to be the beginning of the "low carb craze." During the late 1990s and early 2000s low-carbohydrate diets became some of the most popular diets in the U.S. (by some accounts as much as 18% of the population was using a low-carbohydrate diet at its peak) and spread to many countries. These were, in fact, noted by some food manufacturers and restaurant chains as substantially affecting their businesses (notably Krispy Kreme, and many fast food chains). This was in spite of the fact that the mainstream medical community continued to denounce low-carbohydrate diets as being a dangerous trend. It is, however, valuable to note that many of these same doctors and institutions at the same time quietly began altering their own advice to be closer to the low-carbohydrate recommendations (e.g. eating more protein, eating more fiber/less starch, reducing consumption of juices by children). The low carbohydrate advocates did some adjustments of their own increasingly advocating controlling fat and eliminating trans fat. Many of the diet guides and gurus that appeared at this time intentionally distanced themselves from Atkins and the term low carb (because of the controversies) even though their recommendations were based on largely the same principles (e.g. the Zone diet). As such it is often a matter of debate which diets are really low-carbohydrate and which are not. The 1990s and 2000 also saw the publication of an increased number of clinical studies regarding the effectiveness and safety (pro's and con's) of low-carbohydrate diets.
Tuesday, October 02, 2007
Chemical Reaction
A chemical reaction is a process which ends in the interconversion of chemical subtances. The substance or substances involved are called reactants. These chemical reactions are usually chracterised by a chemical change a usually yield one or more products. Classically, these changes strictly involve the motion of electrons in forming and breaking of chemical bonds. Though the concept of a chemical reaction, in the particular notion of a chemical equasion is applicable to transformations of elementary particles, as well as nuclear reactions.
Different chemical reactions are used with chemical sythesis in order to get a desired product. In biochemistry, series of chemical reactions catalyzed by enzymes form metabolic pathways, by which syntheses and decompositions ordinarily impossible in conditions within a cell are performed.
Different chemical reactions are used with chemical sythesis in order to get a desired product. In biochemistry, series of chemical reactions catalyzed by enzymes form metabolic pathways, by which syntheses and decompositions ordinarily impossible in conditions within a cell are performed.
Tuesday, January 16, 2007
Riboflavin Vitamin G
Riboflavin (E101), also known as vitamin B2 or vitamin G, is an easily absorbed micronutrient with a key role in maintaining health in animals. Like the other B vitamins, it supports energy production by aiding in the metabolising of fats, carbohydrates and proteins. Vitamin G is also required for red blood cell formation, respiration, antibody production and for regulating human growth and reproduction. It is essential for healthy skin, nails, hair growth and general good health, including regulating thyroid activity. Riboflavin also helps in the prevention or treatment of many types of eye disorders, including some cases of cataracts. It may assist bloodshot, itching or burning eyes and abnormal sensitivity to light.
Milk, cheese, leafy green vegetables, liver, yeast, almonds and mature soybeans are good sources of vitamin B2, but exposure to light will destroy the riboflavin in these natural sources. Any excess is excreted in the urine, frequently imparting a bright yellow color. As the human body does not store riboflavin it is thought deficiency is common.
In processed foods it is very likely to have been produced synthetically using genetically modified Bacillus subtilis, altered to both increase the bacteria's production of riboflavin and to introduce an antibiotic (ampicillin) resistance marker.
Riboflavin is yellow or orange-yellow in colour and in addition to being used as a food colouring it is also used to fortify some foods. It can be found in baby foods, breakfast cereals, sauces, processed cheese, fruit drinks and vitamin-enriched milk products as well as being widely used in vitamin supplements.
It is difficult to incorporate riboflavin into many liquid products because it has poor solubility in water. Hence the requirement for E101a riboflavin-5'-phosphate, a more expensive but more soluble form of riboflavin.
Milk, cheese, leafy green vegetables, liver, yeast, almonds and mature soybeans are good sources of vitamin B2, but exposure to light will destroy the riboflavin in these natural sources. Any excess is excreted in the urine, frequently imparting a bright yellow color. As the human body does not store riboflavin it is thought deficiency is common.
In processed foods it is very likely to have been produced synthetically using genetically modified Bacillus subtilis, altered to both increase the bacteria's production of riboflavin and to introduce an antibiotic (ampicillin) resistance marker.
Riboflavin is yellow or orange-yellow in colour and in addition to being used as a food colouring it is also used to fortify some foods. It can be found in baby foods, breakfast cereals, sauces, processed cheese, fruit drinks and vitamin-enriched milk products as well as being widely used in vitamin supplements.
It is difficult to incorporate riboflavin into many liquid products because it has poor solubility in water. Hence the requirement for E101a riboflavin-5'-phosphate, a more expensive but more soluble form of riboflavin.
Monday, September 11, 2006
Coenzyme Q10 Energy Catalyst
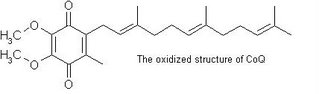
Coenzyme Q10 acts as a catalyst for energy production, which increases metabolism level at a cellular level. It is known to support heart health and overall cellular vigor. CoQ10 levels diminish with age and supplementation can help restore energy, vitality. Coenzyme Q10 (also called CoQ10 or ubiquinone) is also involved in maintaining normal heart function, and it is an antioxidant, scavenging free radicals throughout the body.
CoQ10 is required by every cell in our body and is the key to chemical reactions that produce cellular energy. Just as an automobile engine with poor spark plugs chokes, sputters and dies, without enough CoQ10 (our cellular spark plug) our health can break down, resulting in all kinds of degenerative diseases. Studies show that CoQ10 may be effective for congestive heart failure, high blood pressure, some forms of cancer, and gum disease.
Thursday, March 02, 2006
Vitamin C Ascorbic Acid

Vitamin C is a water-soluble nutrient essential for life, used by the human body for many purposes. To the best of scientific knowledge, all animals and plants synthesize their own vitamin C, except for a small number of animals, including guinea pigs, humans, apes, the red-vented bulbul, a fruit-eating bat and a species of trout, that are not able to. This, along with the related fact that man possesses three of the four enzymes that animals employ to manufacture the substance in relatively large amounts, has led researchers such as Irwin Stone and Linus Pauling to hypothesize that man once manufactured this substance in the body millions of years ago in quantities roughly estimated at 3-4,000 mg daily, but later lost the ability to do this through evolution. If true, this would of course mean that vitamin C was misnamed as a vitamin and is in fact a vital macronutrient like fat or carbohydrate.
Vitamin C was first isolated in 1928, and in 1932 it was proved to be the agent which prevents scurvy. Albert Szent-Györgyi was awarded the Nobel Prize for this feat.
Vitamin C is a weak acid, called ascorbic acid or ascorbate (an L-enantiomer of ascorbic acid; an l-enantiomer is simply one of two mirror image forms of the same chemical molecular structure, see optical isomers). The active part of the substance is the ascorbate ion, which can express itself as either an acid or a salt of ascorbate that is neutral or slightly basic. Commercial vitamin C is often a mix of ascorbic acid, sodium ascorbate and/or other ascorbates.
Saturday, September 03, 2005
Vitamin E as Antioxidant
Vitamin E is also known as Tocopherol. Alpha-tocopherol is traditionally recognized as the most active form of vitamin E in humans, and is a powerful antioxidant. The measurement of "vitamin E" activity in international units (IU) was based on fertility enhancement by the prevention of spontaneous abortions in pregnant rats relative to alpha tocopherol. It increases naturally to about 150% of normal in the maternal circulation during human pregnancies. The other isomers are slowly being recognized as research begins to elucidate their additional roles in the human body. Many naturopathic and orthomolecular medicine advocates suggest that vitamin E supplements contain at least 20% by weight of the other natural vitamin E isomers. Commercially available blends of natural vitamin E include "mixed tocopherols" and "high gamma tocopherol" formulas. Also selenium, Coenzyme Q10, and ample vitamin C have been shown to be essential cofactors of natural tocopherols.
Antioxidants such as vitamin E act to protect cells against the effects of free radicals, which are potentially damaging by-products of the body's metabolism. Free radicals can cause cell damage that may contribute to the development of cardiovascular disease and cancer. Vitamin C and other anti-oxidants recycle vitamin E end-products back into effective suppressors of free radicals. Studies are underway to determine whether vitamin E might help prevent or delay the development of those chronic diseases.
Vegetable oils, nuts, wheat germ and green leafy vegetables are the main dietary sources of vitamin E. Fortified breakfast cereals are also an important source of vitamin E in the United States. Although originally extracted from wheat germ oil, most natural vitamin E supplements are now derived from vegetable oils, usually soybean oil.
Commercial vitamin E supplements can be classified into several distinct categories: fully synthetic vitamin E, "d,l-alpha-tocopherol", the most inexpensive, most commonly sold supplement form usually as the acetate ester; semisynthetic "natural source" vitamin E esters, the "natural source" forms used in tablets and multiple vitamins; highly fractionated natural d-alpha tocopherol; less fractionated "natural mixed tocopherols"; high gamma-tocopherol fraction supplements; and tocotrienol supplements.
Synthetic vitamin E, usually marked as d,l-tocopherol or d,l tocopheryl acetate, with 50% d-alpha tocopherol moiety and 50% l-alpha-tocopherol moiety, as synthesized by an earlier process is now actually manufactured as all-racemic alpha tocopherol, with only about one alpha tocopherol molecule in 8 molecules as actual d-alpha tocpherol. The synthetic form is not as active as the natural alpha tocopherol form. The 1950's thalidomide disaster with numerous severe birth defects is a common example of d- vs l- epimer forms type problem with synthesized racemic mixtures. Information on any side effects of the synthetic vitamin E epimers is not readily available. Naturopathic and orthomolecular medicine advocates have long considered the synthetic vitamin E forms to be with little or no merit for cancer, circulatory and heart diseases.
Semisynthetic "natural source" vitamin E, manufacturers convert the common natural beta, gamma and delta tocopherol isomers into esters using acetic or succinic acid and add methyl groups to yield d-alpha tocopheryl esters such as d-alpha tocopheryl acetate or d-alpha tocopheryl succinate. These tocopheryl esters are more stable and are easy to use in tablets and multiple vitamin pills. Because only alpha tocopherols were officially counted as "vitamin E" in supplements, refiners and manufacturers faced enormous economic pressure to esterify and methylate the other natural tocopherol isomers, d-beta-, d-gamma- and d-delta-tocopherol into d-alpha tocopheryl acetate or succinate. In the healthy human body, the semisynthetic forms are easily de-esterified over several days, primarily in the liver, but not for common problems in aged or ill patients.
Antioxidants such as vitamin E act to protect cells against the effects of free radicals, which are potentially damaging by-products of the body's metabolism. Free radicals can cause cell damage that may contribute to the development of cardiovascular disease and cancer. Vitamin C and other anti-oxidants recycle vitamin E end-products back into effective suppressors of free radicals. Studies are underway to determine whether vitamin E might help prevent or delay the development of those chronic diseases.
Vegetable oils, nuts, wheat germ and green leafy vegetables are the main dietary sources of vitamin E. Fortified breakfast cereals are also an important source of vitamin E in the United States. Although originally extracted from wheat germ oil, most natural vitamin E supplements are now derived from vegetable oils, usually soybean oil.
Commercial vitamin E supplements can be classified into several distinct categories: fully synthetic vitamin E, "d,l-alpha-tocopherol", the most inexpensive, most commonly sold supplement form usually as the acetate ester; semisynthetic "natural source" vitamin E esters, the "natural source" forms used in tablets and multiple vitamins; highly fractionated natural d-alpha tocopherol; less fractionated "natural mixed tocopherols"; high gamma-tocopherol fraction supplements; and tocotrienol supplements.
Synthetic vitamin E, usually marked as d,l-tocopherol or d,l tocopheryl acetate, with 50% d-alpha tocopherol moiety and 50% l-alpha-tocopherol moiety, as synthesized by an earlier process is now actually manufactured as all-racemic alpha tocopherol, with only about one alpha tocopherol molecule in 8 molecules as actual d-alpha tocpherol. The synthetic form is not as active as the natural alpha tocopherol form. The 1950's thalidomide disaster with numerous severe birth defects is a common example of d- vs l- epimer forms type problem with synthesized racemic mixtures. Information on any side effects of the synthetic vitamin E epimers is not readily available. Naturopathic and orthomolecular medicine advocates have long considered the synthetic vitamin E forms to be with little or no merit for cancer, circulatory and heart diseases.
Semisynthetic "natural source" vitamin E, manufacturers convert the common natural beta, gamma and delta tocopherol isomers into esters using acetic or succinic acid and add methyl groups to yield d-alpha tocopheryl esters such as d-alpha tocopheryl acetate or d-alpha tocopheryl succinate. These tocopheryl esters are more stable and are easy to use in tablets and multiple vitamin pills. Because only alpha tocopherols were officially counted as "vitamin E" in supplements, refiners and manufacturers faced enormous economic pressure to esterify and methylate the other natural tocopherol isomers, d-beta-, d-gamma- and d-delta-tocopherol into d-alpha tocopheryl acetate or succinate. In the healthy human body, the semisynthetic forms are easily de-esterified over several days, primarily in the liver, but not for common problems in aged or ill patients.
Subscribe to:
Posts (Atom)